Headquartered in Caesarea, Israel, HYDROLITE is one of the industrial partners directly involved in the ECO2Fuel project. As the global demand for hydrogen solutions starts to grow exponentially – in recognition of the critical role of hydrogen as a carbon-free fuel in a zero-carbon economy – HYDROLITE’s superior Anion exchange membrane (AEM) technology offers a compelling solution that combines the cost advantages of liquid Alkaline Fuel Cells and Electrolyzers, with the high performance of solid-state Proton Exchange Membrane devices.
Over the past decade HYDROLITE has developed extensive, proprietary AEM materials technology, pilot-scale production, and device operation capabilities, and holds an extensive IP portfolio of 70 patent cases with more in the pipeline.


In the ECO2Fuel project, Hydrolite contributes to the following areas:
- Investigating membrane and ionomer requirements in a CO2 Electrolyzer environment
- Providing optimized membranes and ionomers to fit requirements
- Scaling up membrane production and size
The membrane is at the heart of electrolyser. Bearing multiple roles for the reliable and durable operation of the device, the membrane must excel in the following:
- High ionic conductivity
- Gas tight to H2, O2, CO2 and other gaseous products (crossover)
- Mechanical properties
- Low swelling
- Chemical stability under harsh conditions (temperature, pH, oxidation)
As compared to H2 Electrolyzer, the CO2 Electrolyzer developed in the ECO2Fuel project poses new challenges due to the harsher conditions, in particular the need for the membrane to sustain the presence of carbonates (showing lower conductivity than hydroxide anions) as well as hydrocarbons and alcohols, some of which are often used to dissolve the membrane during production.

Hydrolite uses various strategies to handle those difficulties, such as using materials with known tolerance to alcohols and hydrocarbons as well as, crosslinking to reduce swelling and avoid slow dissolution. This in turn causes challenges in terms of adaptation of the fabrication process and scaleup. Hydrolite successfully implemented manufacturing process to produce membranes of large size showing high barrier properties without compromising on ion conductivity and chemical stability. This was achieved by fine tuning the molecular structure, amount of cationic groups, crosslinking degree and addition of non-conducting polymers to obtain all the desired characteristics. Hydrolite provides ECO2Fuel with ionomers and membranes tailored for the target application.
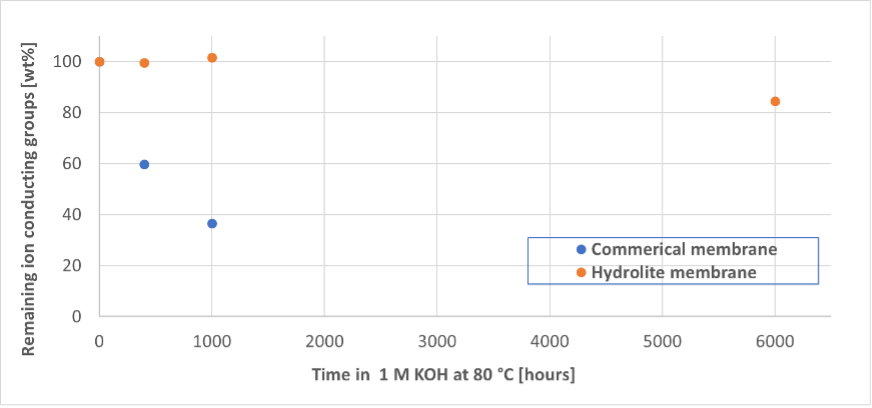
Using only hydrocarbon-based ionomers and membranes, Hydrolite avoids the use of environmentally dangerous perfluorinated polymers as used in other electrolyzers, in line with long term objectives of ECO2Fuel for a sustainable future.
Looking toward the future, Hydrolite plans to further develop and establish the manufacturing process and facilities to supply membranes in large scales and footprints, exhibiting satisfactory uniformity and mechanical robustness with high ionic conductivity.






