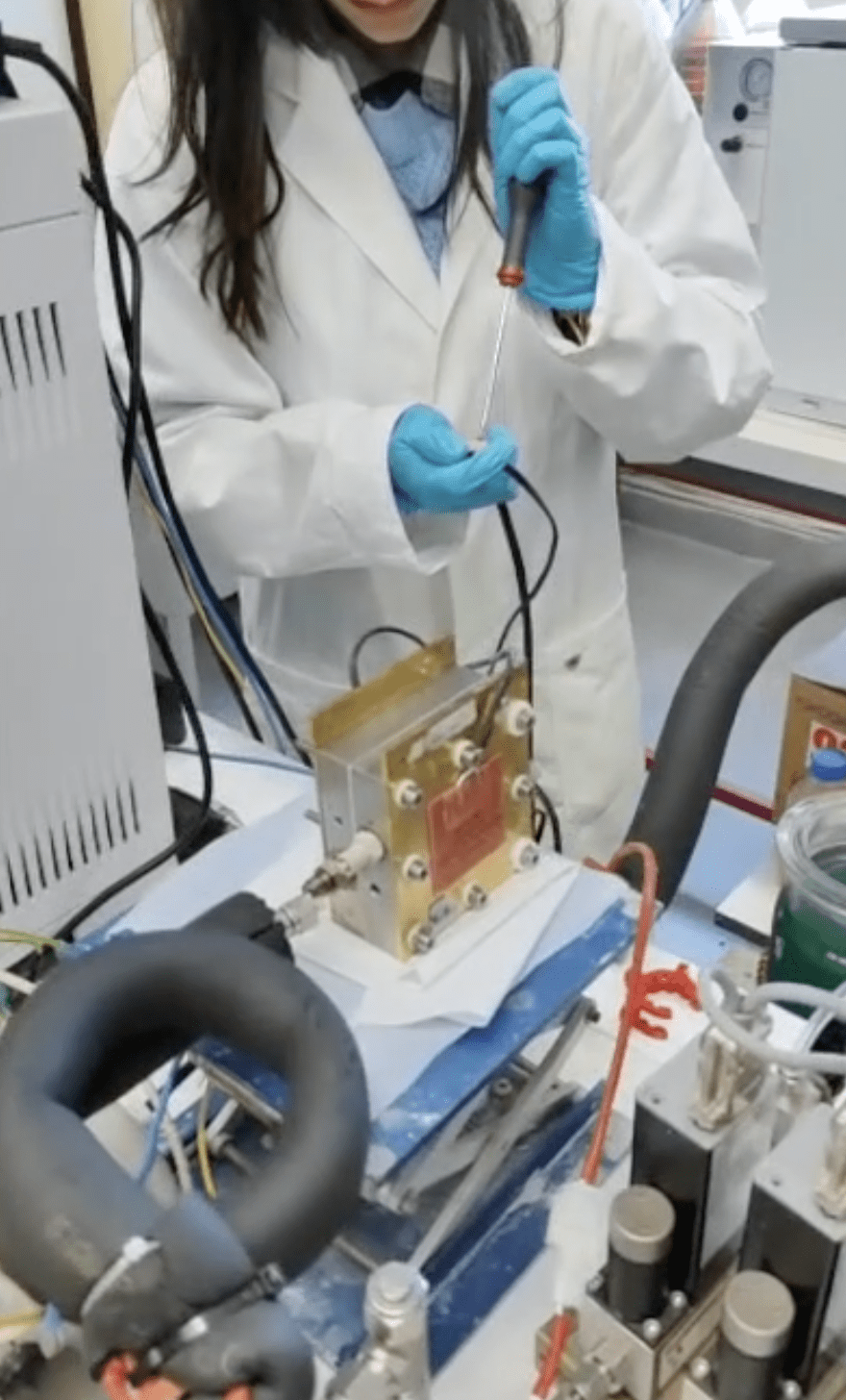
Studying CO2 conversion into e-fuels, especially inside a system resembling real-world applications, includes many steps.
CNR-ITAE, a research institute in Messina, Italy, and consortium partner of the ECO2Fuel project, show us these steps, emphasizing the labour-intensive research needed to develop green technologies such as the ECO2Fuel CO2 electrolyser.
Minute 0-0:08: The first step is to prepare the catalyst-coated electrodes – For that, the catalyst for the reactions that convert CO2 into e-fuels is coated on electrodes are prepared by spraying the catalyst from a liquid dispersion onto the electrode surface.
The second step (not shown on the clip but essential for the process) is the preparation of the so-called membrane-electrode assembly (MEA) consisting of an anion-exchange membrane sandwiched between the two catalyst-coated electrodes, one for the anode side and one for the cathode side.
Minute 0:09-0:27: The MEA is then placed into the electrochemical cell with a zero-gap design, meaning that the water and the CO2 are being pumped through the cell with a very close contact to the MEA with the advantage of a very small ohmic resistance, hence zero-gap.
The zero-gap cell is then connected to the water and CO2 supply and to the electrical power that will derive the electrochemical reactions inside the cell.
Minute 0:28-1:06: The liquid products, such as ethanol and propanol, are collected in a cold trap and analysed by a headspace gas chromatograph coupled with a mass spectrometer at the end of the reaction. In contrast, gaseous products such as methane, ethylene, and carbon monoxide are analysed continuously (not on the clip) by an online gas chromatograph.