A Deep Dive into Energy Conversion and Storage
Energy conversion and storage are pivotal to a sustainable society that harnesses renewable energy sources such as solar and wind power. At the Department of Energy Conversion and Storage, Technical University of Denmark (DTU), we are at the forefront of developing electrolysis, fuel cells, batteries, thermal energy storage, and other power-to-X technologies. Our group’s primary focus is on low-temperature electrochemical systems, including polymer electrolyte fuel cells, alkaline electrolysers, flow batteries, and carbon capture and CO2 reduction. Our research encompasses the development and characterization of functional materials and components, as well as single cells, stacks, and systems.
The Role of Anion-Exchange Membranes in CO2 Reduction
Anion-exchange membranes (AEMs) are the heart of CO2 reduction reaction (CO2RR) electrolysers, serving as charge carrier conductors and electrode separators. Their performance and durability significantly impact cell performance. As part of the ECO2Fuel project, DTU is investigating various electrolyte materials provided by our ECO2Fuel partner, Hydrolite. The investigation focuses on evaluating their mechanical stability, ion conductivity, and gas crossover, particularly CO2, by diffusion and migration. The lab setups for these evaluations are briefly described below.
Evaluating Mechanical Stability
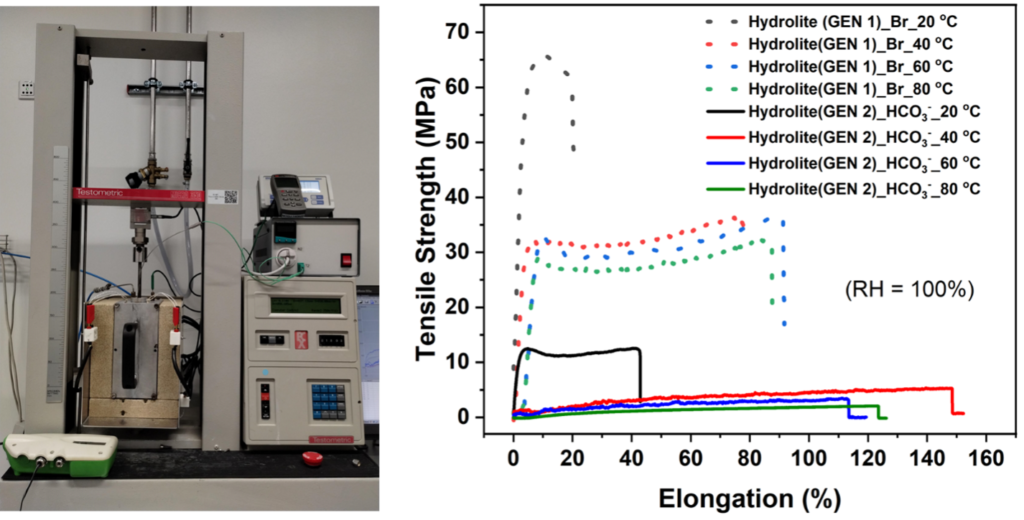
The AEMs’ satisfactory mechanical properties are crucial for handling membrane electrode assembly (MEA) and ensuring cell durability. Therefore, we conducted mechanical stability tests to examine the membranes’ stress-strain.
The setup for the tensile strength measurements was modified (Instron 3344) to include an additional chamber constructed around the membrane sample, equipped with heating elements for temperature control and an inlet and outlet for humidified air to achieve varying atmospheric humidity levels. A humidity sensor was installed next to the membrane sample to monitor temperature and humidity during tensile strength measurements.
Assessing Ion Conductivity
The ion conductivity of the membrane, especially under ambient conditions, is a key factor influencing an electrochemical cell’s performance. We measured the anion (OH-, HCO3-, CO3-2) conductivities of the membranes using a four-probe electrochemical alternating current impedance spectroscopy method with a Scribner B-112 over a frequency range of 100 mHz to 1 MHz. A rectangular membrane was sandwiched between platinum electrodes, and conductivity was measured from 20 °C to 80 °C at 30-minute intervals under hydrous conditions.

Understanding Gas Crossover
The CO2 electrolysis process faces a significant challenge related to the carbonation of the electrolyte, a consequence of performing CO2RR in alkaline conditions to suppress the H2 evolution reaction. The parasitic reactions of CO2 with the alkaline electrolytes result in bicarbonate precipitation and flooding in gas diffusion electrodes, CO2 crossover to the anode, low carbon utilization efficiencies, and additional costs for CO2 and electrolyte recycling. These issues seriously hinder the scale-up and commercialization of CO2 electrolysers.
To address these challenges, as part of the ECO2Fuel project, DTU aims to investigate the carbon crossover mechanisms and factors influencing CO2 crossover in electrolysers in terms of the concentration of the electrolytes (anolyte and/or catholyte), the CO2 supply rate, and cell configuration.
Device Setup and Crossover Measurement
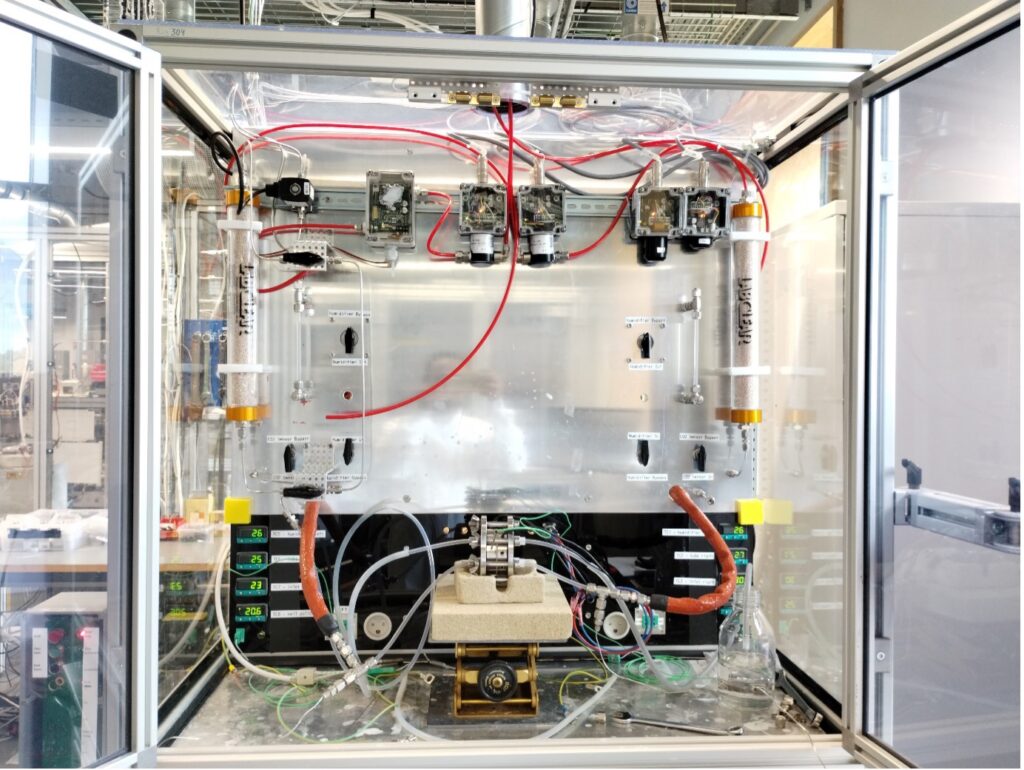
The DTU’s test rig for CO2 crossover flow cell and the hardware for providing the gas mixture and analysing the exhaust are shown in Figure 1. The outlet gas composition on the anode side was controlled by mass flow controllers (MFCs).
The total CO2 gas flow rate was measured using a CO2 sensor. Prior to membrane electrode assembly (MEA), membranes were washed with deionized water to remove excess electrolyte. Membrane and gas diffusion electrode (GDEs) were pressed into a cell with gaskets and graphite flow fields. The cell was connected to a modified alkaline electrolyser stand with temperature and pressure control. MFCs were used to supply humidified N2 and CO2 on the cathode side and argon (Ar) on the anode side. A potentiostat was used to apply current and take impedance measurements for determining the cell resistance. Outlet gases on both sides were analysed for CO2 concentration using a CO2 sensor. The sensors were calibrated to ranges of 0-1000 and 0-10000 ppm of CO2.