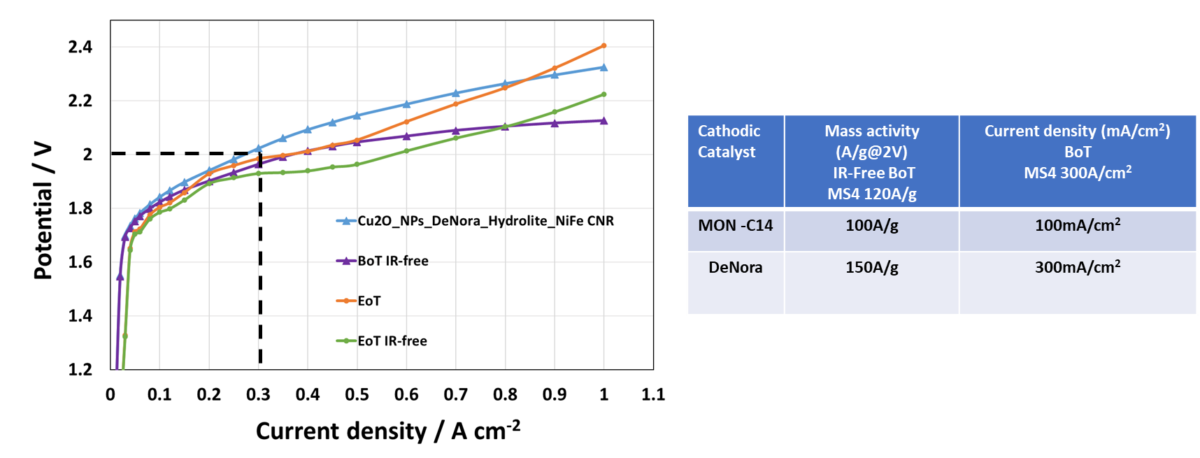
In the progressive world of CO2 Electrolysis, milestones MS9 and MS10 mark significant advancements, albeit with unique challenges. The recent achievement in MS9 was notable, as the cell potential reached an impressive 300mA/cm2 at 2V per cell. This indicates a substantial improvement in efficiency and performance, albeit not fully meeting the set milestone.
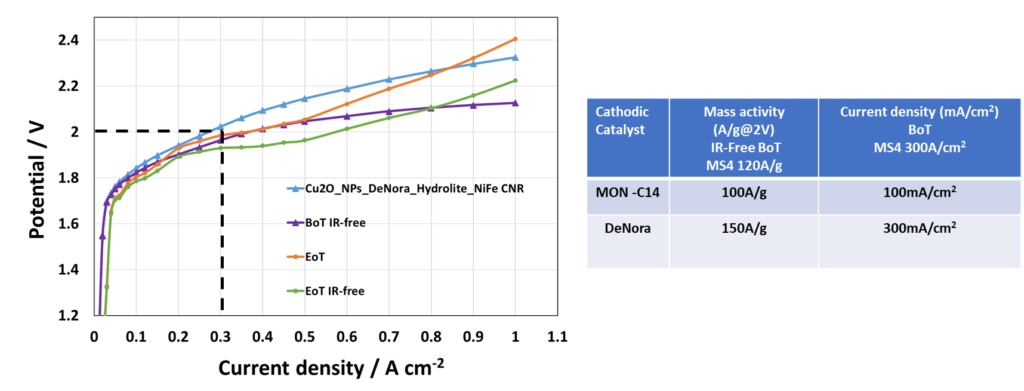
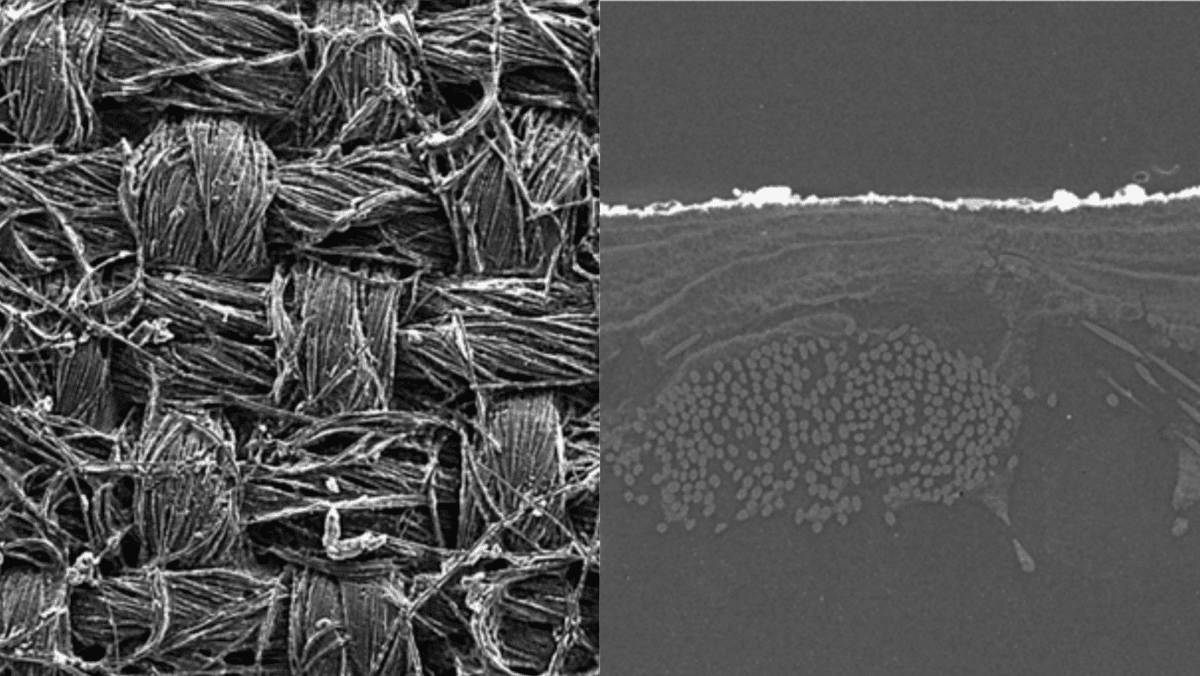
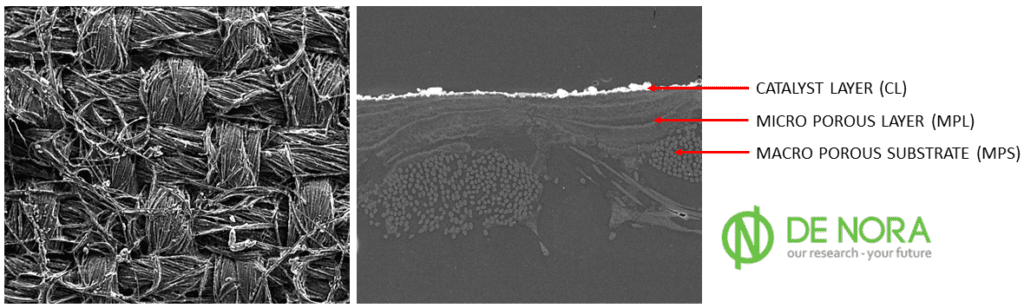
Turning to MS10, significant strides were made in material selection. The milestone involved the critical selection and freezing of Gas Diffusion Layers (GDLs) and catalysts for both anode and cathode sides. This decision is crucial as it lays the foundation for the future efficiency and reliability of the fuel cells.
However, these advancements didn’t come without their challenges. One of the primary issues encountered was the stability of the test in terms of operational hours. This was primarily due to the clogging of the flow field, a result of salt precipitation. This phenomenon poses a significant threat to the consistent operation and longevity of the fuel cells.
In response to these challenges, researchers and engineers are diligently studying new procedures to mitigate these issues. Developing strategies to avoid clogging and ensure uninterrupted operation is a top priority. Another obstacle that surfaced was related to the scaling up of the substrate and the supplying materials. This highlights the complexities involved in transitioning from laboratory-scale successes to large-scale, commercially viable solutions.
Despite these challenges, the progress made in milestones MS9 and MS10 is a testament to the ongoing innovation in CO2 Electrolysis. As solutions to these challenges are developed, we can expect further advancements in this promising field, paving the way for more sustainable and efficient energy solutions.
Source: CNR & DeNora