Hydrolite has continuously improved its anion exchange membrane, reaching several important milestones. Ethanol crossover has been reduced by an order of magnitude, from 12 mA/cm2 to 0.3 mA/cm2 (70 °C, 0.5 M in 1 M OH) as shown in Figure 1.
As ethanol is one of the desired products from the CO2 electrolysis, it is crucial to prevent from crossing over the membrane.
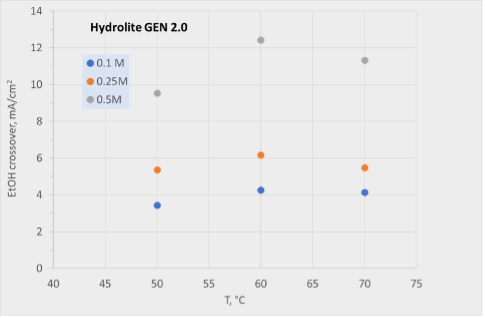
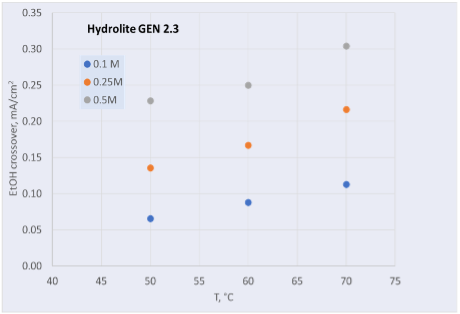
Figure 1: Ethanol crossover versus temperature for older generation (left) and newer generation (right) membranes produced at Hydrolite.
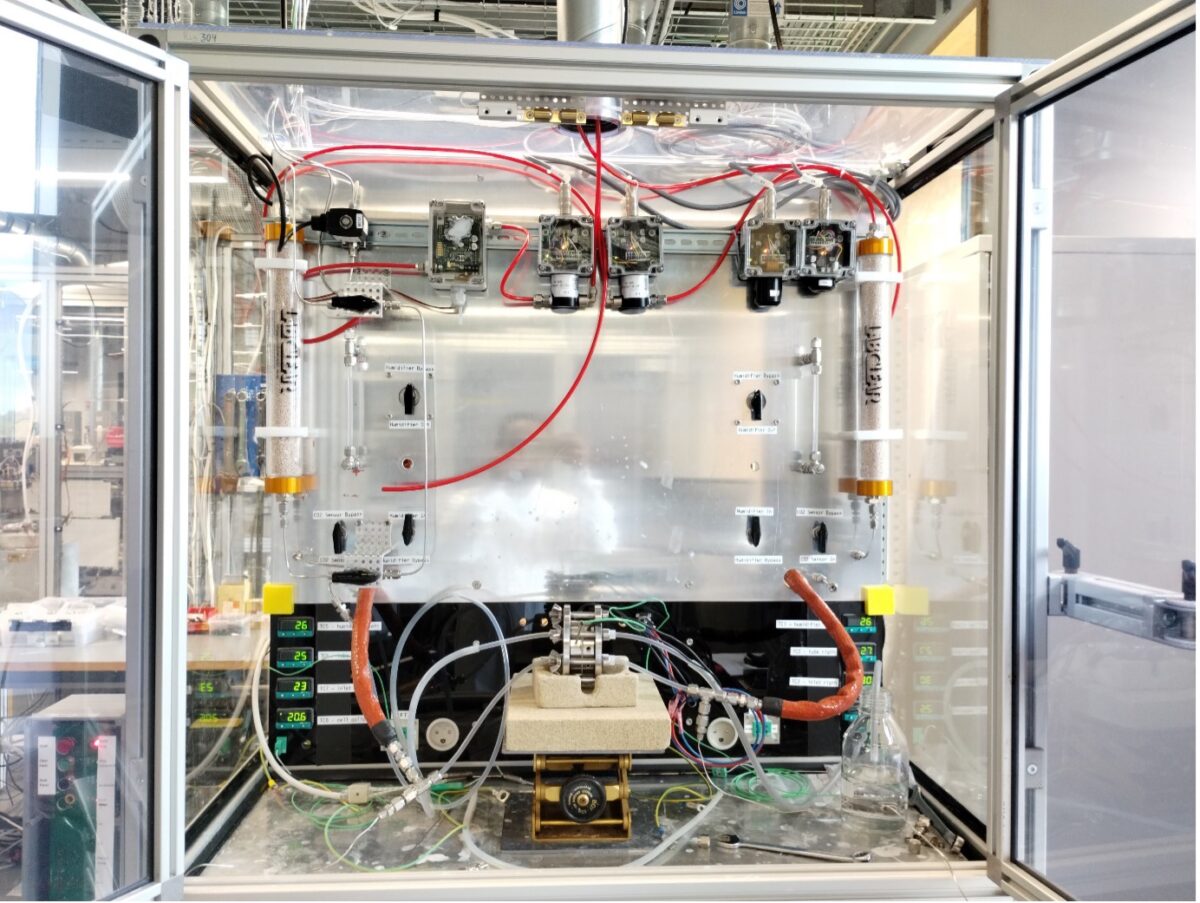
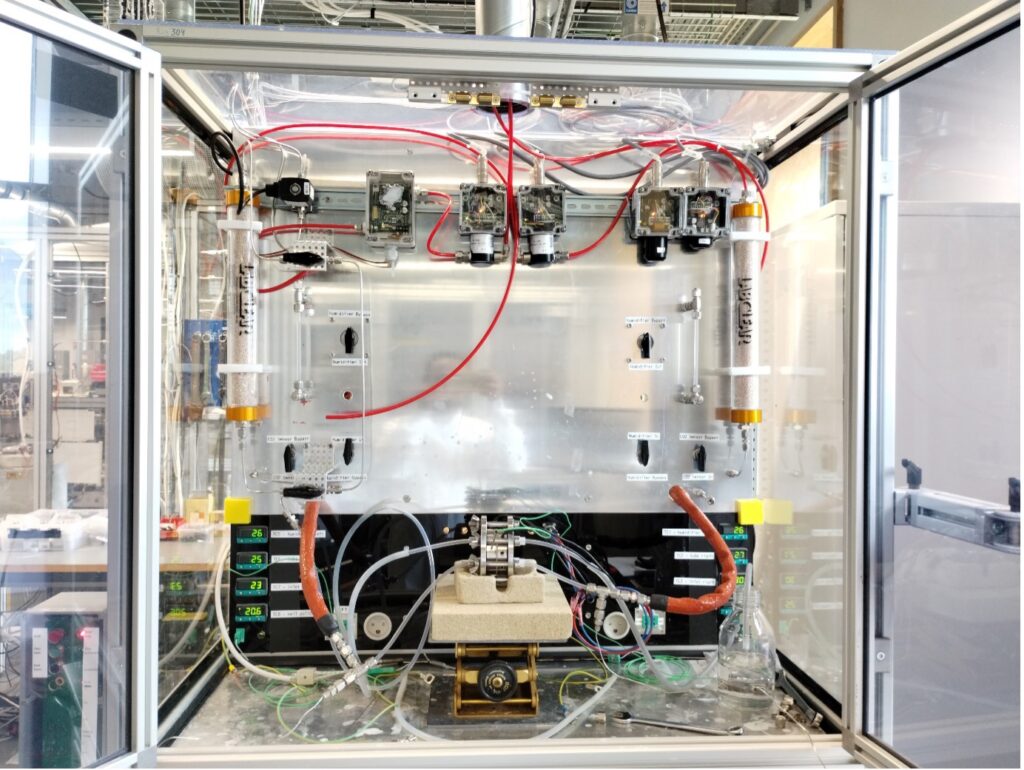
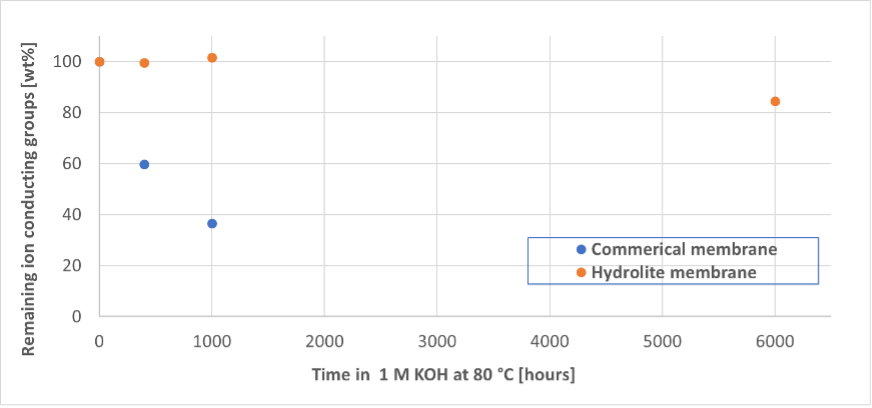
Using a setup specially developed at DTU, CO2 crossover could be measured for various membranes. It is now understood that CO2 crossover increases significantly with increasing current, meaning the CO2 is mainly driven by ionic current crossing the membrane from the cathode to the anode. It was shown the Hydrolite membrane displays lower crossover of CO2 than a commercially available membrane as showed in Figure 2.
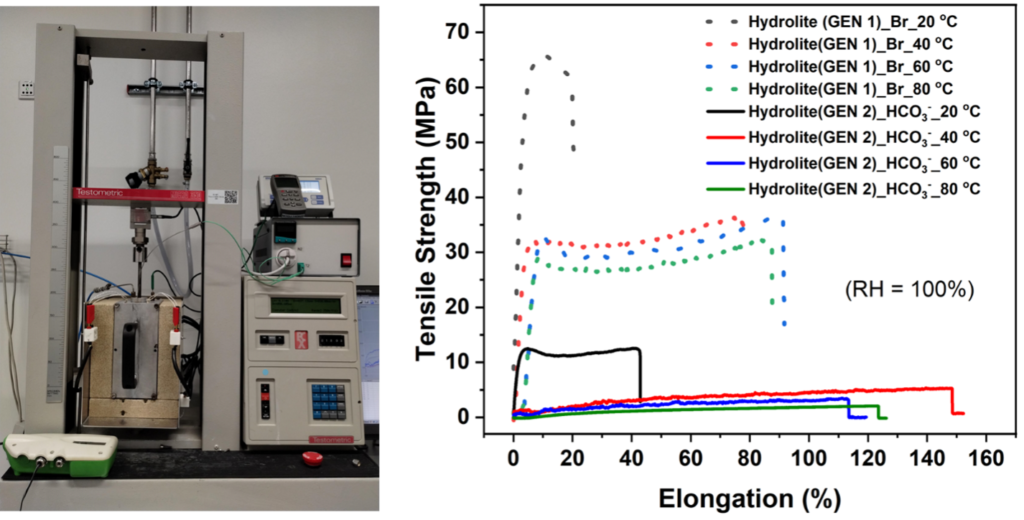
It is important to mention that those improvements were achieved without compromising the hydroxide conductivity of the membrane (> 150 mS/cm at 60 C, in-plane, 100 %RH). One remaining challenge is reaching the mechanical tensile strength target of 20 MPa at 60 °C. Recent developments have shown an improvement of the tensile strength of Hydrolite membranes from 4 to 17 MPa, hence approaching the target. We are confident that this milestone will be reached soon.
On the fabrication side, Hydrolite delivered to its partners about 10 m2 of large size membrane for the CO2 electrolyser prototype. Significant efforts are being invested to further scale up the membrane fabrication for the supply of 100 m2 of high quality membranes for the demonstrator stack in early 2025.
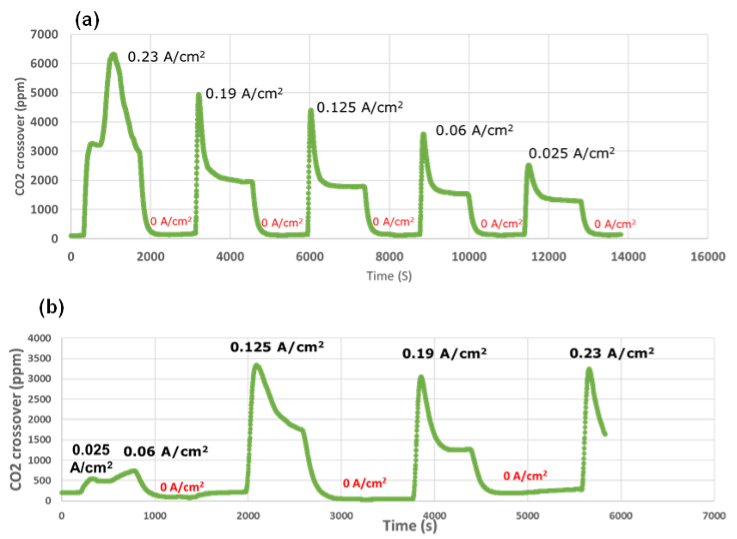
Figure 2: Hydrolite membrane (bottom) showing improved CO2 barrier as compared to commercial membrane (top)