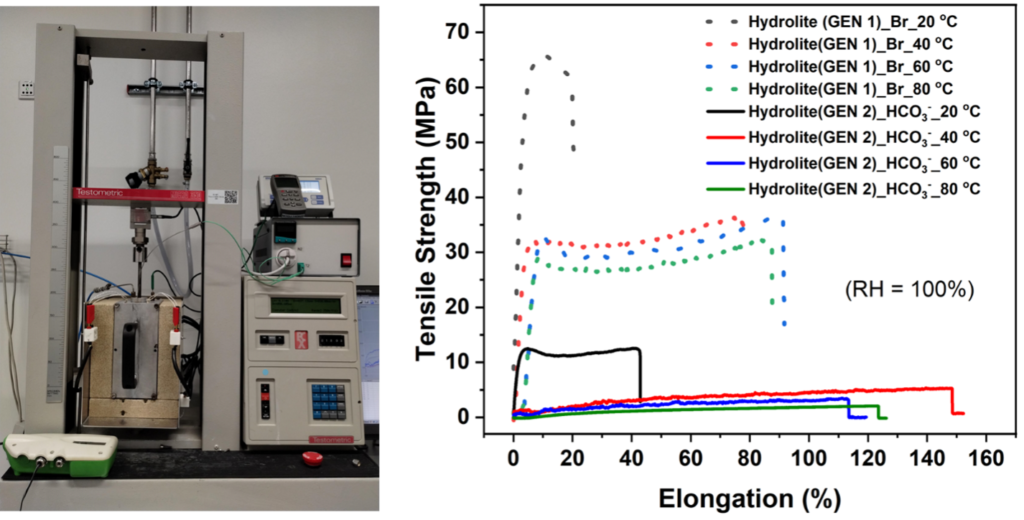
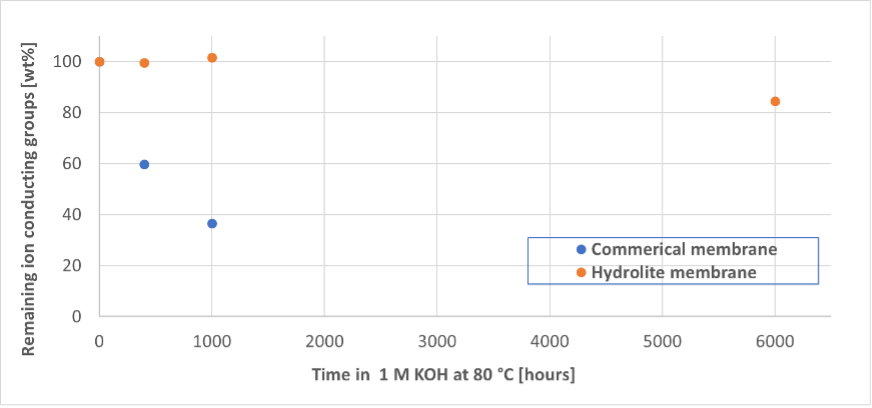
The membrane is at the heart of any electrochemical membrane reactor, with the general tasks of separating the anode and cathode electrodes and chambers from each other while allowing ion conduction to complete the electrochemical circuit.
However, for the ECO2Fuel technology, the membrane must fulfil three additional tasks to allow the efficient conversion of CO2 into green fuels and valuable chemicals:
High ion conduction
The conductivity of ions through the membrane dictates the ohmic resistance and therefore the energy efficiency of the ECO2Fuel technology. High ion conduction means low ohmic resistance, leading to better energy efficiency.
Alcohol exclusion
The ECO2Fuel technology produces among other carbonaceous chemicals also alcohols by combining CO2, water and electrons from renewable sources. However, ion-conducting membranes normally swell in contact with alcohols, allowing them to cross from the cathode to the anode chamber.
Alcohols that cross the membrane to the anode chamber will face the anode electrode, where they will be oxidised back to CO, reducing the efficiency of the technology. Therefore, membranes in the ECO2Fuel technology need to possess ultra-low alcohol crossover.
Salt exclusion
Finally, the ECO2Fuel process relies on a supporting alkaline electrolyte (salt) to maintain optimum conditions for the electrochemical reactions.
This electrolyte is introduced on the anode electrode, whereas the CO2-to-fuel conversion occurs in the cathode. Only the membrane prevents access of salt to the fuel output line, the third critical function of the ECO2Fuel membrane.
Achieving these three tasks together, Ion conduction, alcohol and salt exclusion, is especially challenging.
In ECO2Fuel, Hydrolite directs the membrane development work package and will develop membranes with unique separation properties, allowing high performance and robustness for the ECO2Fuel technology.
To implement molecular-scale and solution-phase sieving effects tailored to the specific species that need to be excluded to achieve the selective passage of the various reactants and products to where they are needed, Hydrolite is taking advantage of their advanced fabrication techniques.
Our academic partners in the consortium – CNR (Italy), DTU (Denmark), DLR (Germany) and UPV (Spain) provide critical feedback in the form of characterization and evaluation of these speciality components.
Hydrolite also works closely with industry partners including Electrolyzer Stack designer Vito (Belgium) to refine specifications – assuring that required separation and sealing quality are achieved, and Membrane-Electrode Assembly developer De Nora (Italy) to help achieve optimal integration between membranes and electrodes, and thus maximizing the overall performance of the Electrolyzer device.
CNR – Consiglio Nazionale delle Ricerche
DTU – Danmarks Tekniske Universitet
DLR – Deutsches Zentrum für Luft- und Raumfahrt
UPV – Universitat Politècnica de València
Dr Miles Page
CTO