The DLR Institute of Engineering Thermodynamics in Oldenburg is mainly involved in two topics of the ECO2Fuel project: the characterization of the membrane and the optimization of the electrodes.
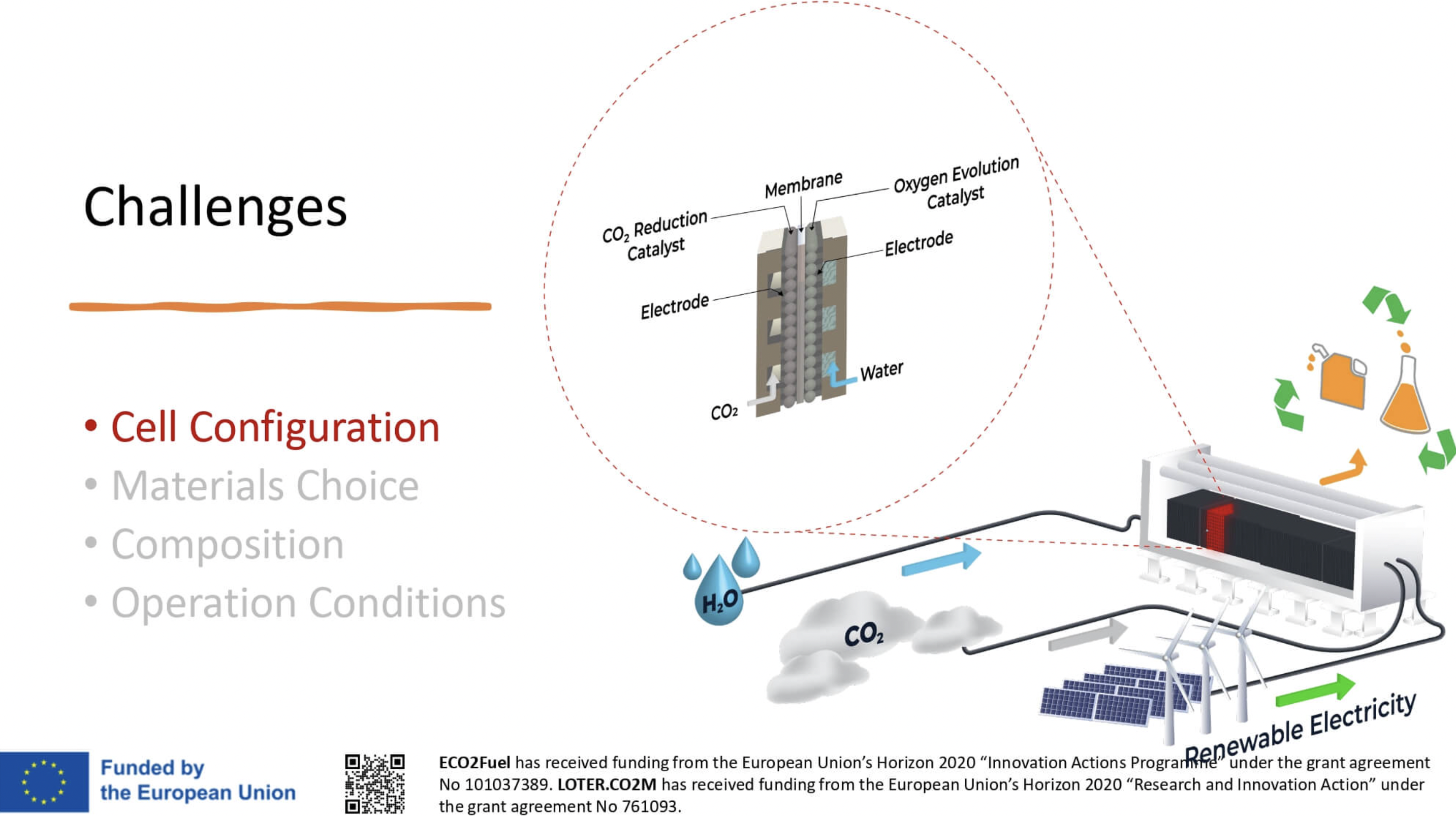
The heart of the electrolysis cell
The membrane is the heart of the cell and responsible for separating the anode and cathode and transporting anions between the two electrodes. The ion-conducting properties of the membrane are a decisive factor in the performance of the electrolysis cell. However, the membrane is also susceptible to chemical or mechanical degradation.
For these reasons, a comprehensive characterization of the performance and stability properties is essential.
These are being investigated using various methods at DLR in Oldenburg, including the dynamic mechanical analysis (DMA).
DMA is a technique where a stress is applied and the strain in the material is analyzed, used to characterize a material’s mechanical properties as a function of the applied stress, temperature, time, atmosphere, or a combination of these parameters.
For the CO2 electrolysis application in ECO2Fuel we investigate the membrane properties in a liquid environment under temperatures from 0 to 100°C. We try to be as close as possible to the operation conditions in the electrolysis cell, which is why the information about the membrane’s properties derived by DMA is crucial for designing the ECO2Fuel’s stack and system.
DLR Oldenburg focuses on developing stable catalyst inks
Regarding electrode optimization, DLR in Oldenburg focuses on developing stable catalyst inks for spray coating on the anode. The catalyst ink consists of the catalyst for the anode reaction, a binder material, and a solvent.
While DLR Oldenburg is not active in developing the catalyst, the materials that accelerate the reaction of CO2 into valuable chemicals, we are focusing on the ink composition.
For the ink, the binder serves has a twofold purpose: it serves as literally a physical binder, holding the catalyst layer (CL) together, on the other hand it is needed as an ion conductor through this layer. That is why the binder in most cases is a polymer material with the same chemical structure as the membrane. Thus, in order to have a good ion conduction, sufficient binder has to be implemented into the CL.
Too much binder leads to a performance decrease since it is blocking the transport of the educts and products through the layer. A well-designed catalyst layer usually has an amount of about 10-40 wt% binder.
The choice of the solvent on the other hand plays an important role for spray coating and the structure of the CL. The solvent is the carrying media in which the catalyst and the binder need to be homogeneously dispersed to allow spraying them onto the electrodes at which the reactions of the ECO2Fuel system will occur.
The ink needs to have a viscosity of maximum 100 mPas, so it can be atomized (sprayed). Isopropanol with a viscosity of 2.4 mPas is well-suited to serve here as a solvent that can easily evaporate during the coating process without applying high temperatures that could damage the membrane or ionomer.
This property allows achieving homogeneous distribution of the catalyst and the binder in the CL, which is critical for achieving high performances in the ECO2Fuel System.
Additionally, surfactants will be added to the ink to increase its stability. Monitoring the stability is done with Dynamic and Electrophoretic Light Scattering (DLS and ELS) and just observing the sedimentation of particles over time inside the catalyst ink.